

Because of the uneven sharing of electrons, one atom will have a slightly negative charge whereas the other atom will have a slightly positive charge. This will result in an unequal distribution of electrons between the two atoms. Therefore, the bond electron pair is pulled more by one atom compared to the other atom, which is participating in making the bond. When the two atoms that form a bond are different, their electronegativities are often different.
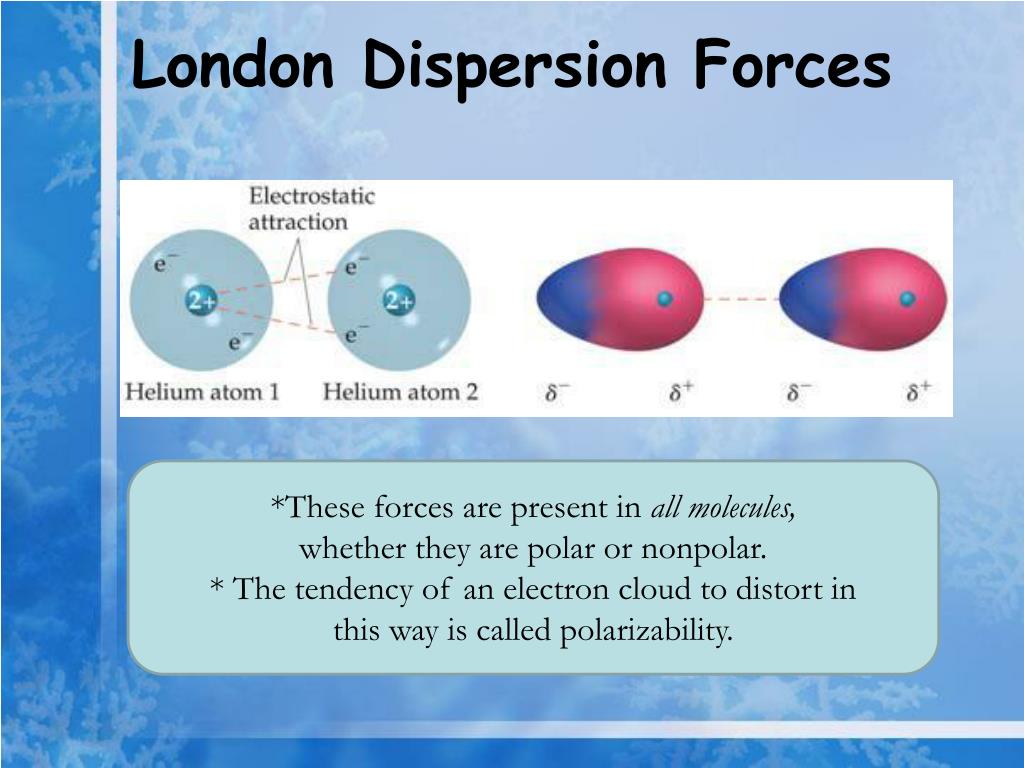

Down the group, the electronegativity values decrease. Therefore, halogens have larger electronegativity values in a period, and group 1 elements have comparatively low electronegativity values. From left to right through a period, the electronegativity value increases. Fluorine has the highest electronegativity value, which is 4 according to the Pauling scale. In the periodic table, there is a pattern as to how the electronegativity values are changing. Usually Pauling scale is used to indicate the electronegativity values. Electronegativity gives a measurement of an atom to attract electrons in a bond. Polarity arises due to the differences in electronegativity. These bonds determine the behavior of molecules. However, all of these intermolecular interactions are weaker than the intramolecular forces like covalent or ionic bonds. Some intermolecular forces are strong while some are weak. Dipole Dipole vs Dispersion | Dipole Dipole Interactions vs Dispersion Forcesĭipole dipole interactions and dispersion forces are intermolecular attractions between molecules.


 0 kommentar(er)
0 kommentar(er)
